👩🔬 Introduction
Our laboratory studies infection immunology and bacterial pathogenesis, focusing on how host factors influence bacterial virulence and immune responses during severe infections. We aim to uncover key molecular and cellular mechanisms in host-pathogen interactions to identify new targets for prevention and treatment.
Our research focuses on three areas: the role of neutrophil extracellular traps (NETs), bacterial extracellular DNA, and bacterial membrane vesicles in biofilm formation and immune regulation. We use tools from immunology, microbiology, molecular biology, and imaging, and collaborate across disciplines.
We welcome students and researchers interested in infection and host-pathogen interactions to join us in exploring the mechanisms behind infectious diseases and developing new therapeutic strategies.

Education
Professional Experience
🔬 Research Interests
- Ongoing research projects include:
- I. The immunopathogenesis of infective endocarditis (IE).
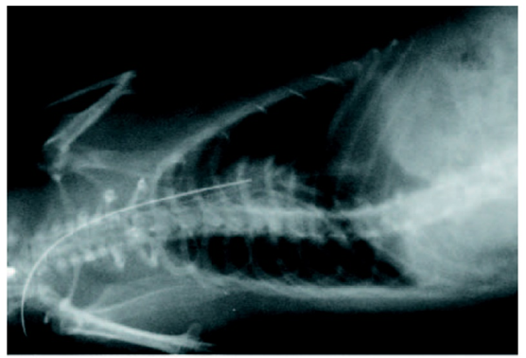
- Fig. 1 The experimental rat model of infective endocarditis
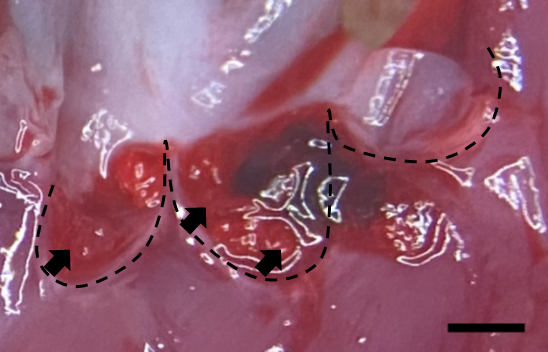
- Fig. 2 The hypothetical model of bacterial colonization on damaged valve in IE
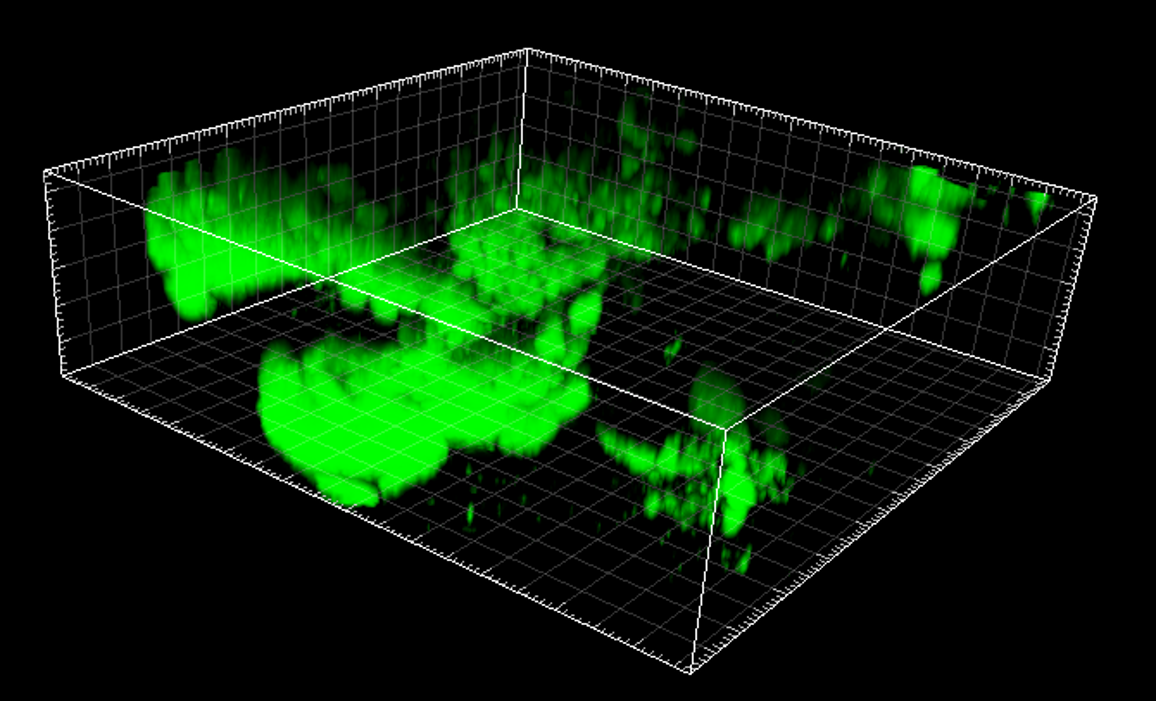
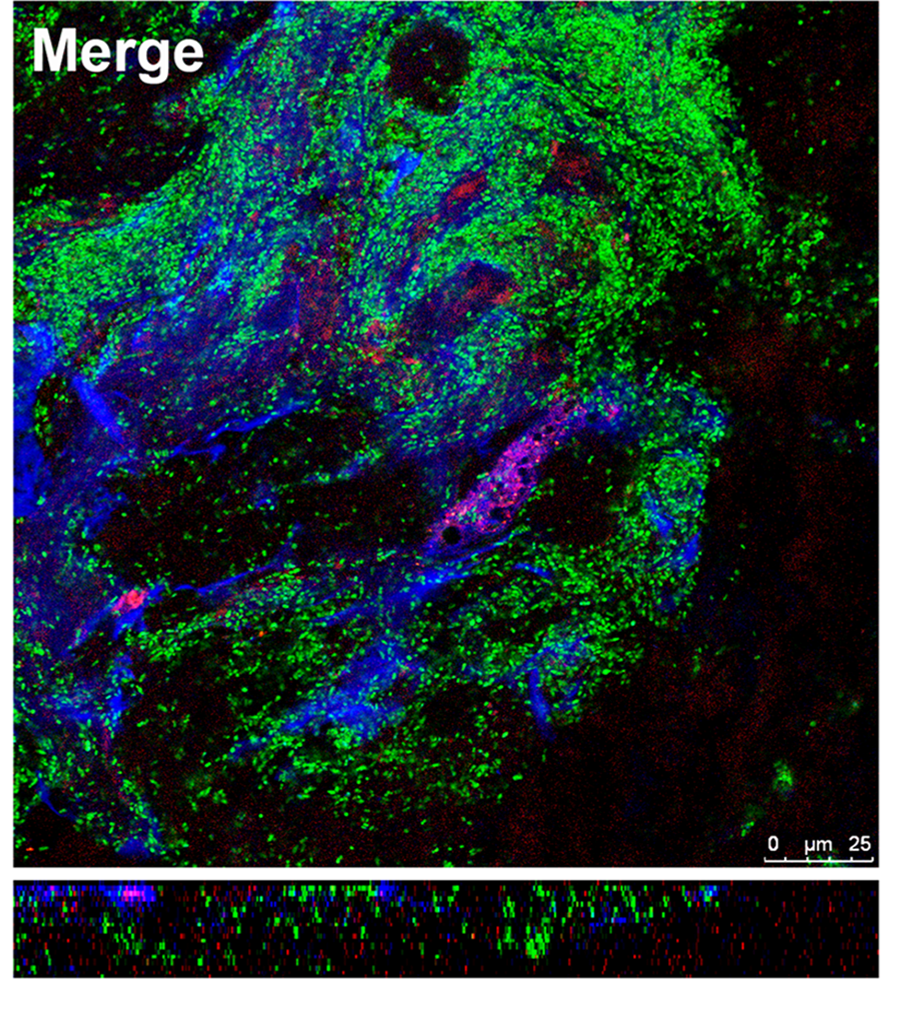
- Fig. 3 The role of NETs in the vegetation maturation in IE
- Fig. 4 The role of AtlA-mediated eDNA and membrane release in biofilm formation in IE
- Fig. 5 The hypothetical mechanisms of eDNA release in S. mutans-induced IE
- II. The roles of oral streptococci in anti-dsDNA antibody production
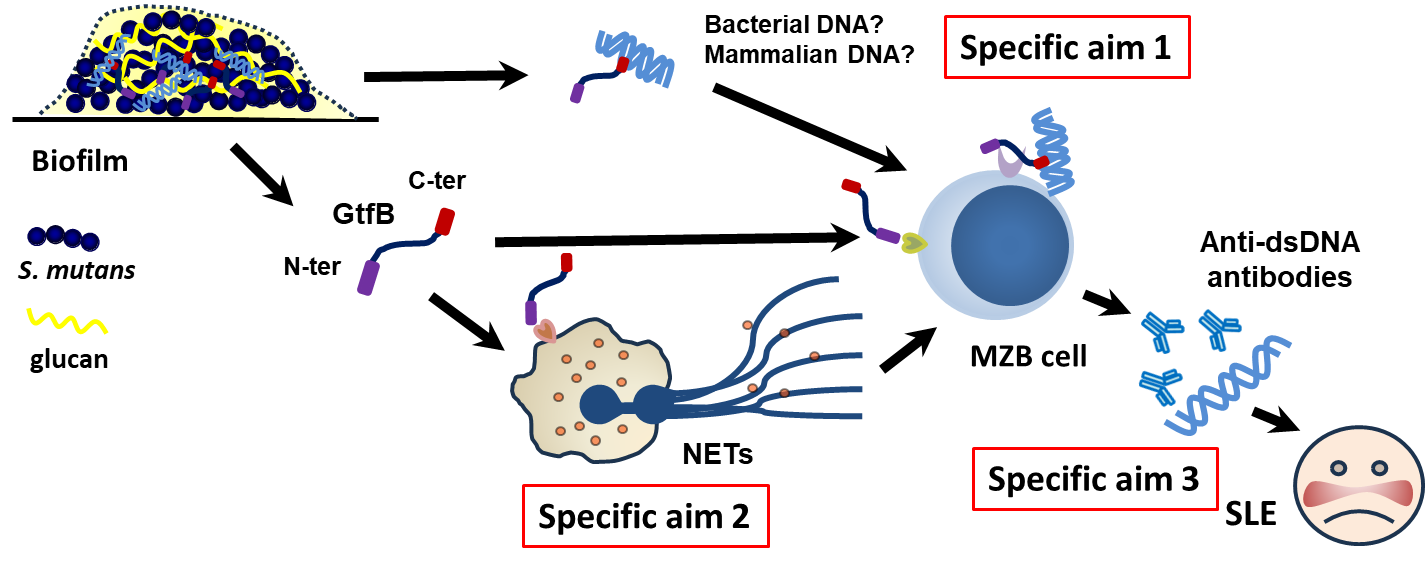
- Fig. 6 The hypothetical mechanism of Gtf-induced anti-dsDNA antibody production
- III. The roles of NETs in other infectious diseases
- 1) Cutibacterium acnes-induced acne vulgaris (in collaboration with Dr. Shi, Shuang Ho Hospital)
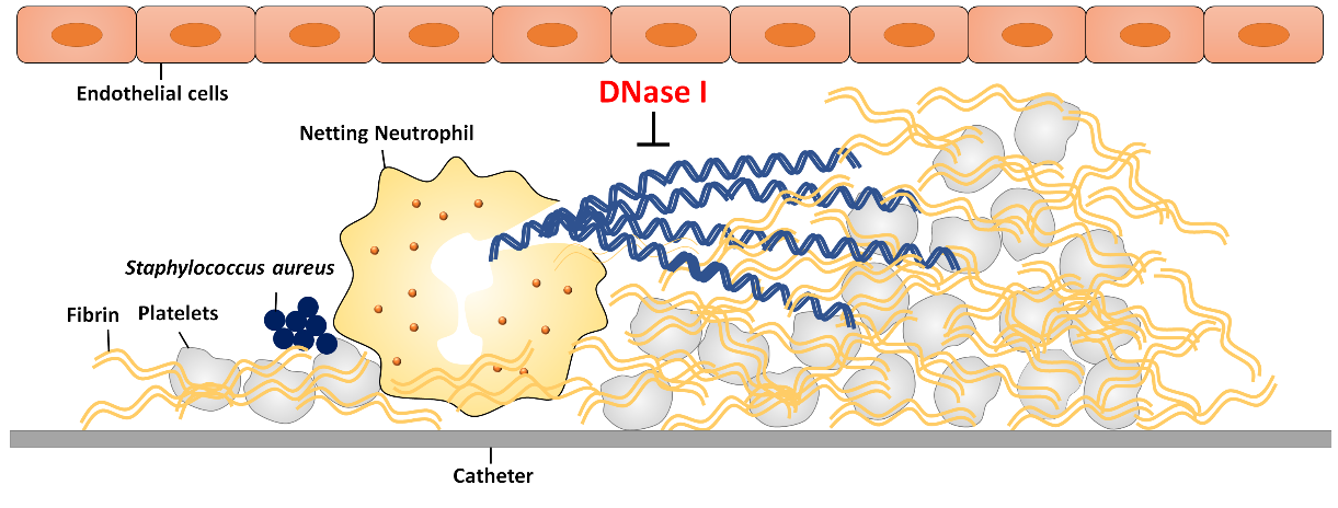
- 2) NETs-associated thrombosis in catheter-related central venous stenosis (In collaboration with Dr. Chen, National Taiwan University Hospital)
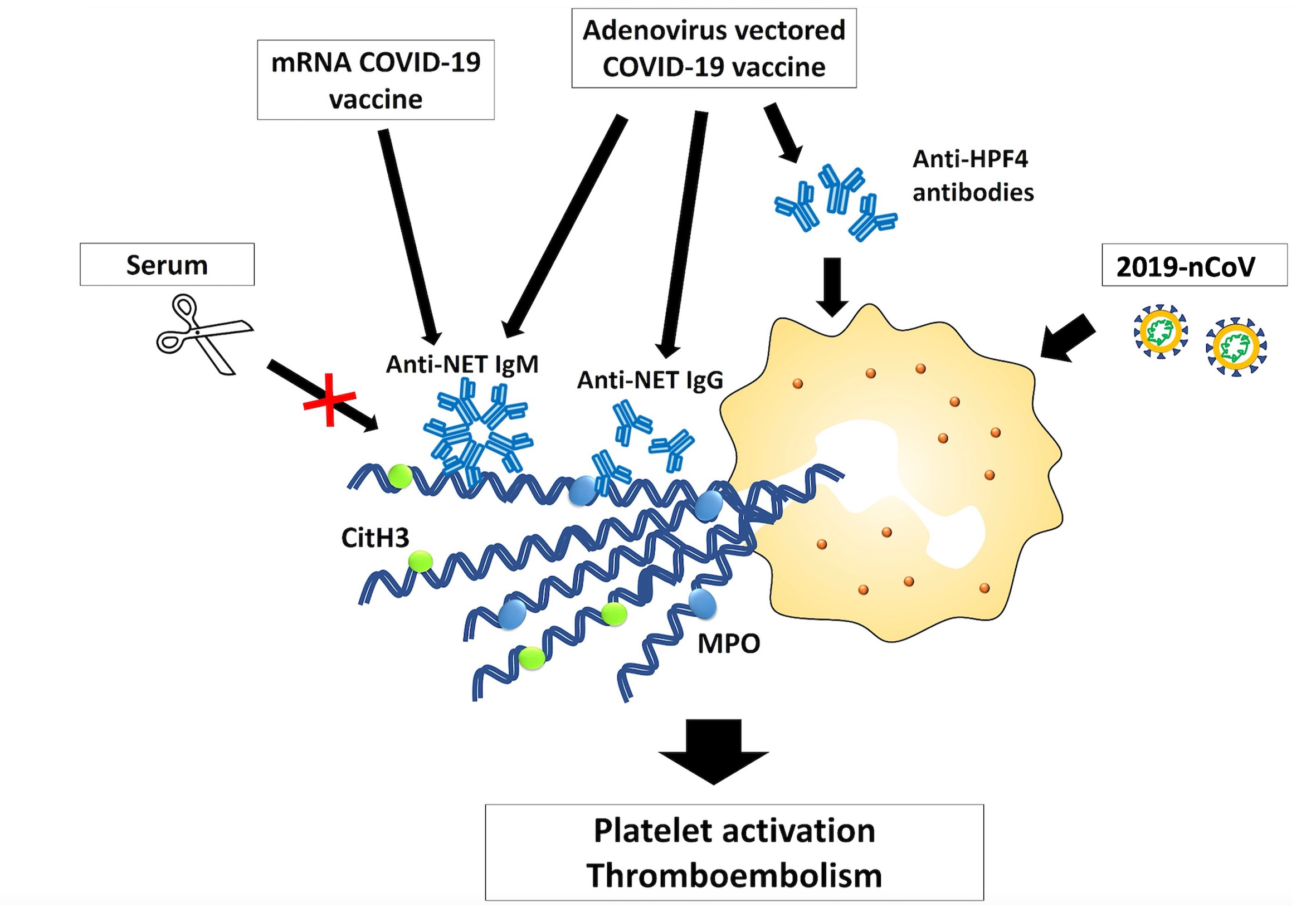
- 3) The role of NETs in vaccine-induced adverse events (AEs) (In collaboration with Dr. Kuo, National Taiwan University Hospital)
- IV. The mechanisms of bacterial antibiotic resistance and identification of alternative therapeutic agents
- 1) Membrane vesicles in the treatment of daptomycin-resistant enterococci (In collaboration with Dr. Chung, National Taiwan University Hospital, and Dr. Hsu, Soochow University)
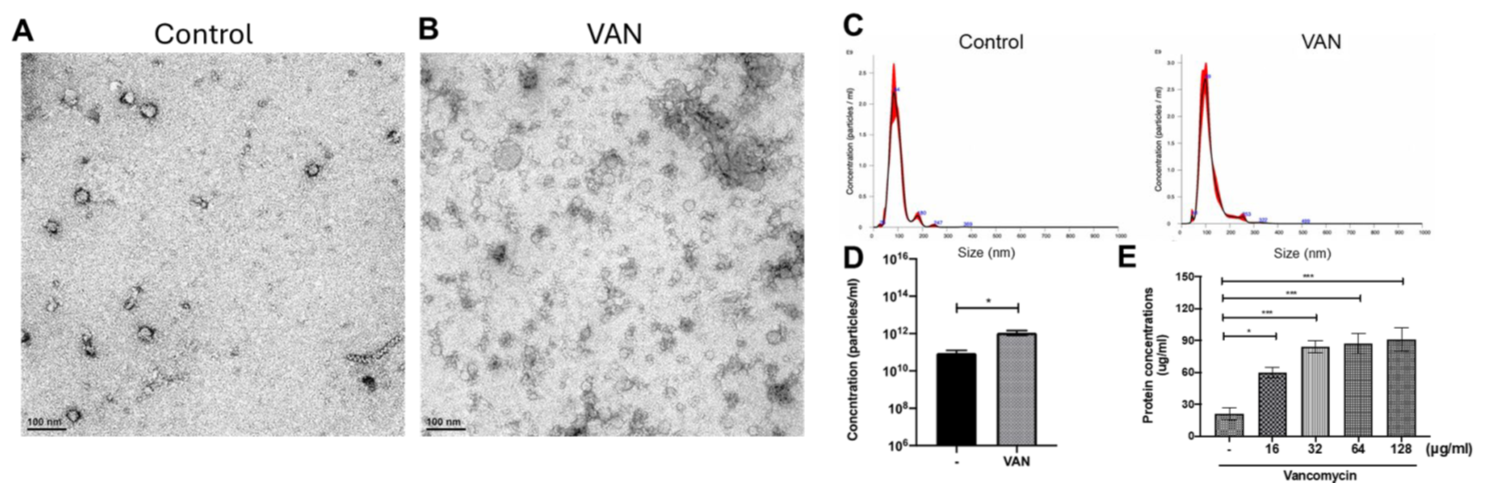
- Fig. 8 Daptomycin treatment enhances MV production of VRE
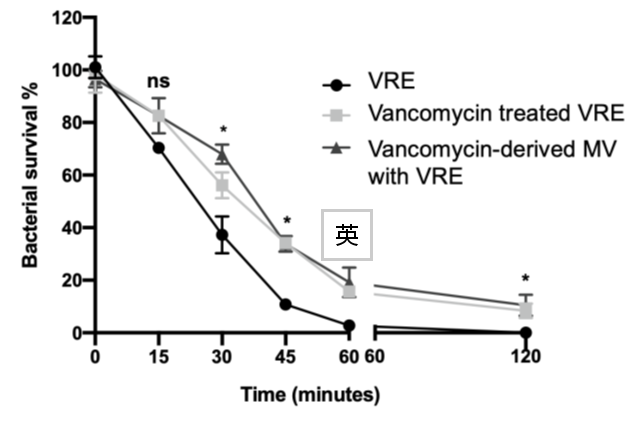
- Fig. 9 MVs enhance bacterial resistance against daptomycin treatment
- 2) Identification of antimicrobial peptides against MRAB and MRKP(In collaboration with Dr. Chung, National Taiwan University Hospital, and Dr. Fan, Taipei Medical University)
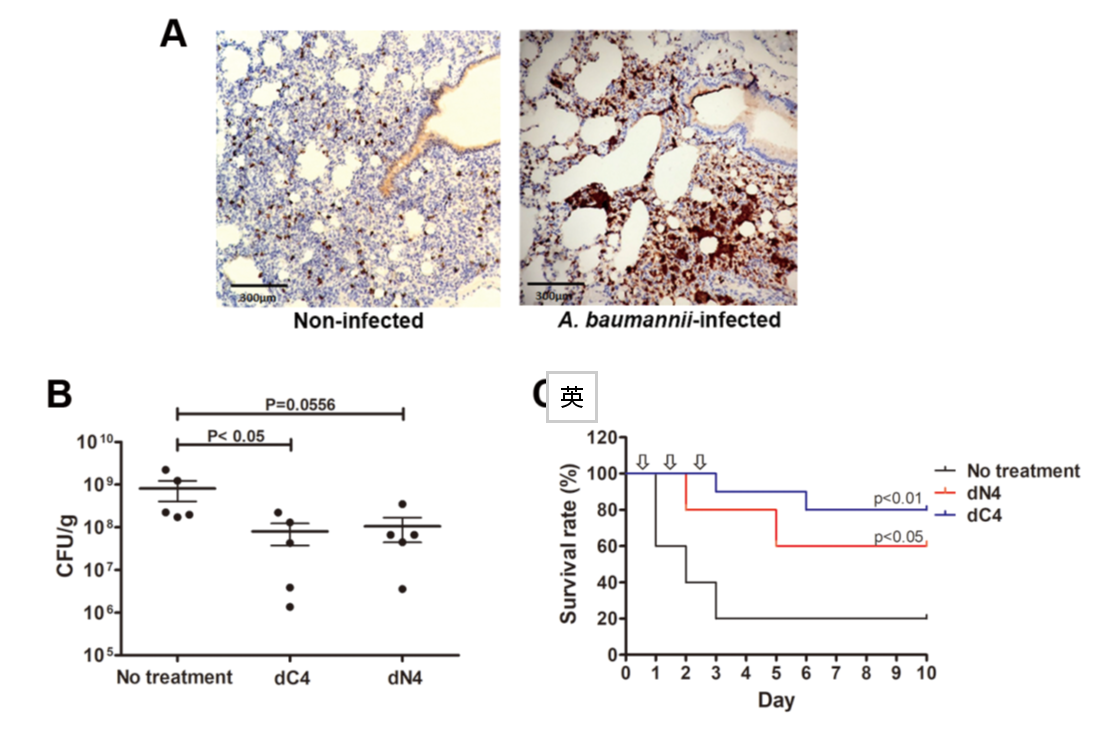
- Fig. 10 AMP dN4 and dC4 exhibit antimicrobial activity in MRAB-induced pneumoniae mouse model.
Host factors play a critical role in regulating bacterial virulence and the progression of systemic lethal diseases. Consequently, targeting the interactions between bacteria and the host represents a promising strategy for developing effective therapies against these infections. The long-term goal of my research is to investigate host-pathogen interactions to uncover novel approaches for managing infectious diseases. In particular, We focus on the roles of neutrophil extracellular traps (NETs), bacterial extracellular DNA, and bacterial membrane vesicles in bacterial pathogenesis. In addition, we investigate the mechanisms underlying bacterial antibiotic resistance and explore alternative agents against antibiotic-resistant pathogens, including vancomycin-resistant enterococci, multidrug-resistant Acinetobacter baumannii, and multidrug-resistant Klebsiella pneumoniae.
Infective endocarditis (IE) is a prototypical biofilm-associated infectious disease of the cardiovascular system, characterized by a high mortality rate (Fig. 1). Clinical management of IE still relies on prolonged administration of high-dose antibiotics and is often complicated by frequent recurrence. My research has demonstrated that host factors significantly enhance the virulence of IE pathogens. For instance, platelets contribute to biofilm formation and antibiotic resistance in IE pathogens (Fig. 2). Furthermore, aggregates formed between bacteria and platelets stimulate infiltrating neutrophils to release neutrophil extracellular traps (NETs), which in turn activate additional platelets and promote thrombus formation. This cascade contributes to the expansion and maturation of IE vegetations (Fig. 3).
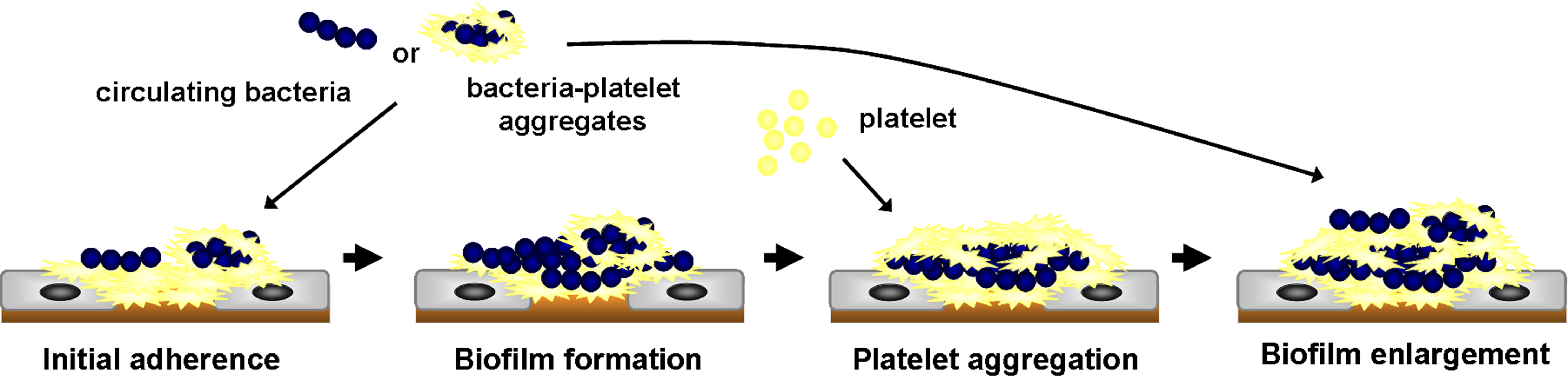
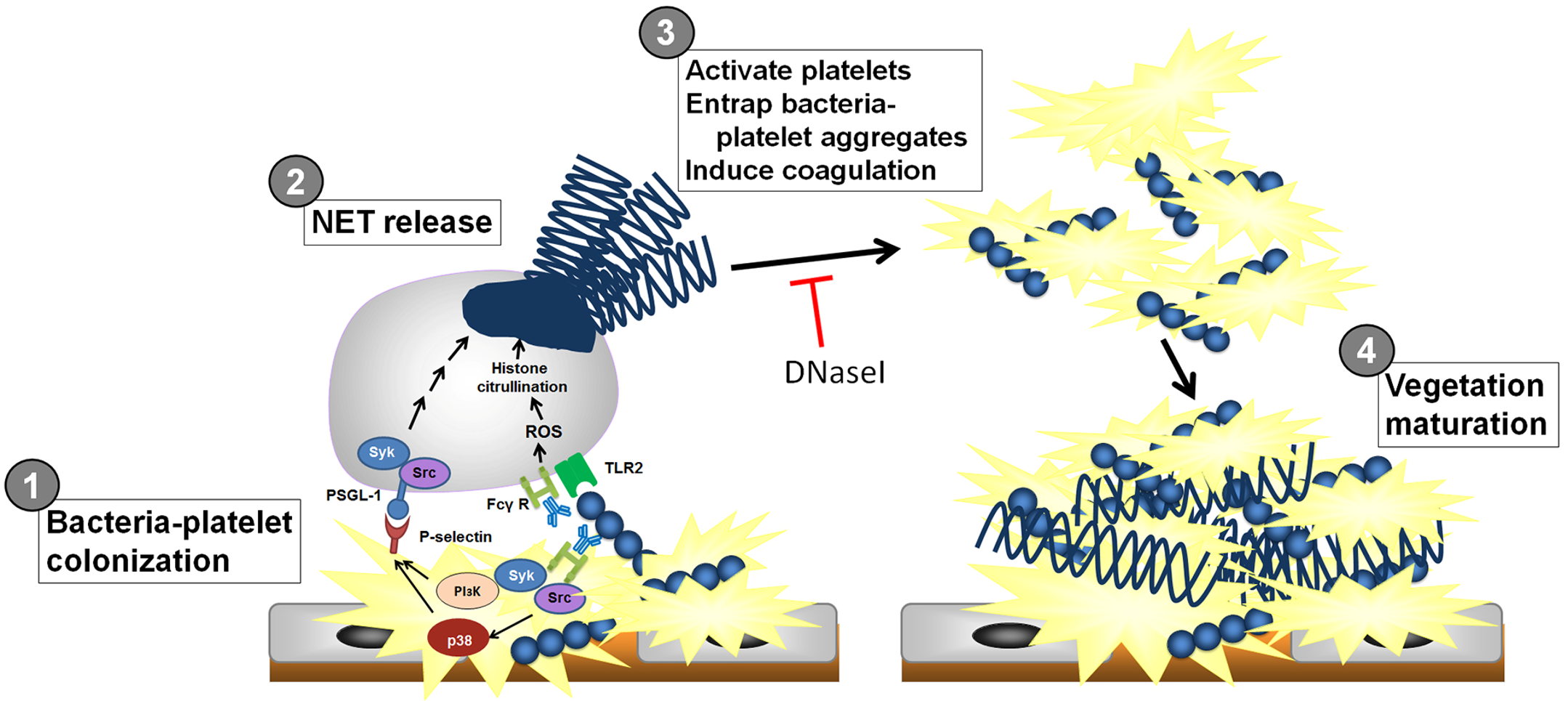
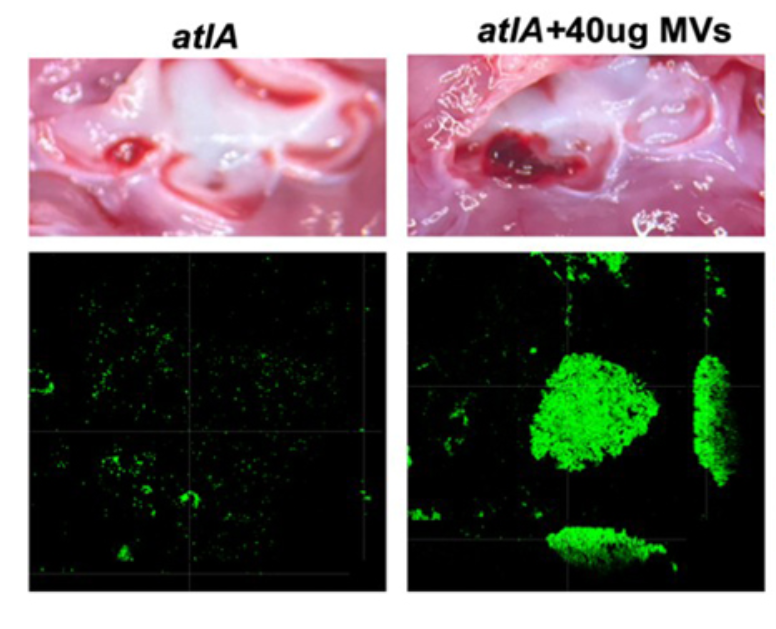
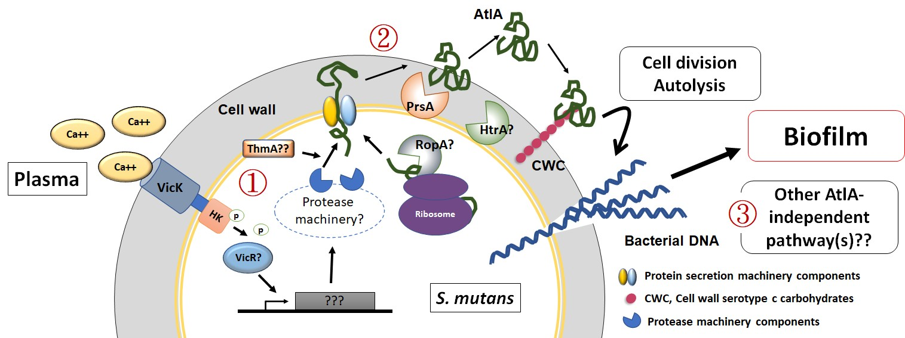
We further demonstrated the critical roles of autolysin (AtlA)-mediated extracellular DNA (eDNA) release and membrane vesicle production in biofilm formation in IE (Fig. 4). In addition, we identified key bacterial virulence factors, including LiaR, PCP, and PrsA, that contribute to eDNA release and membrane vesicle production, both of which promote bacterial biofilm formation in vivo. The underlying molecular mechanisms by which these factors regulate biofilm development will be further investigated (Fig. 5).

Infections are known risk factors for the development of autoimmune diseases. Anti-DNA antibodies, for example, are a key diagnostic marker for systemic lupus erythematosus (SLE) and are thought to contribute to disease pathology. Neutrophil extracellular traps (NETs) are considered a primary source of extracellular DNA and other autoantigens that may trigger autoimmune responses. Our preliminary data show that intravenous infection with oral streptococci or glucosyltransferase (Gtf) leads to the production of anti-DNA antibodies. We hypothesize that oral streptococci within dental biofilms release Gtf, which either binds to extracellular DNA or induces NET formation, thereby promoting anti-dsDNA antibody production and contributing to autoimmune pathogenesis. The underlying mechanisms will be further investigated.
We also investigate the role of neutrophil extracellular traps (NETs) in other infectious diseases in collaboration with clinical physicians.
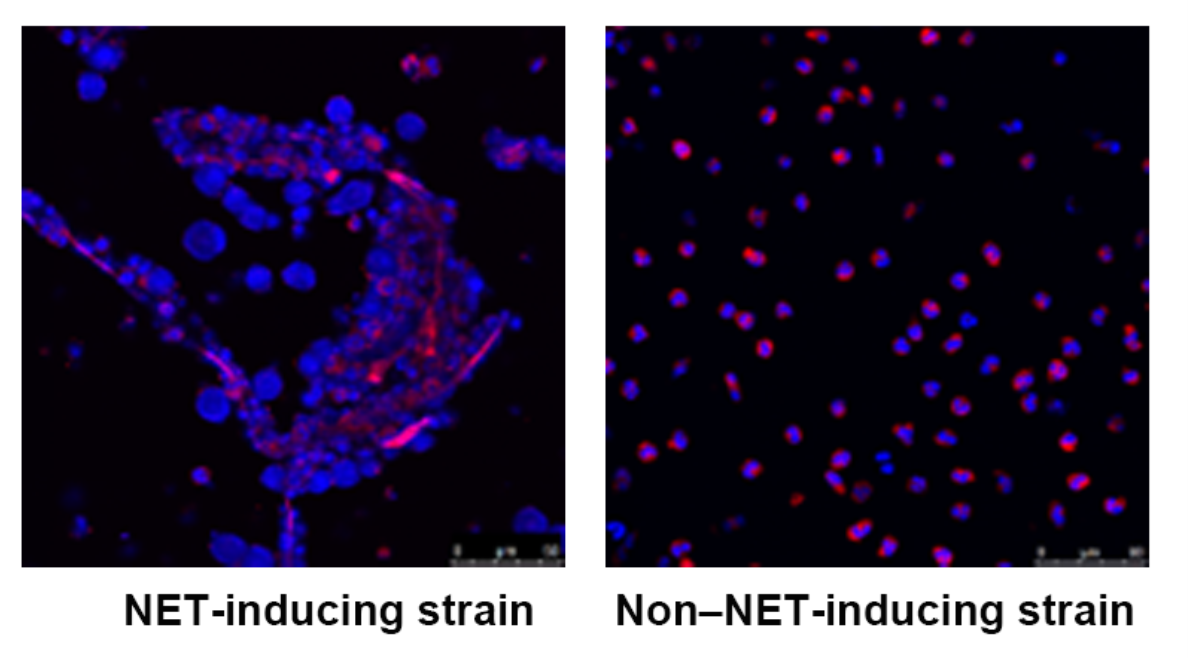
We collect clinical isolates of C. acnes and examine their NET-inducing potential to understand their role in the pathogenesis of acne vulgaris.
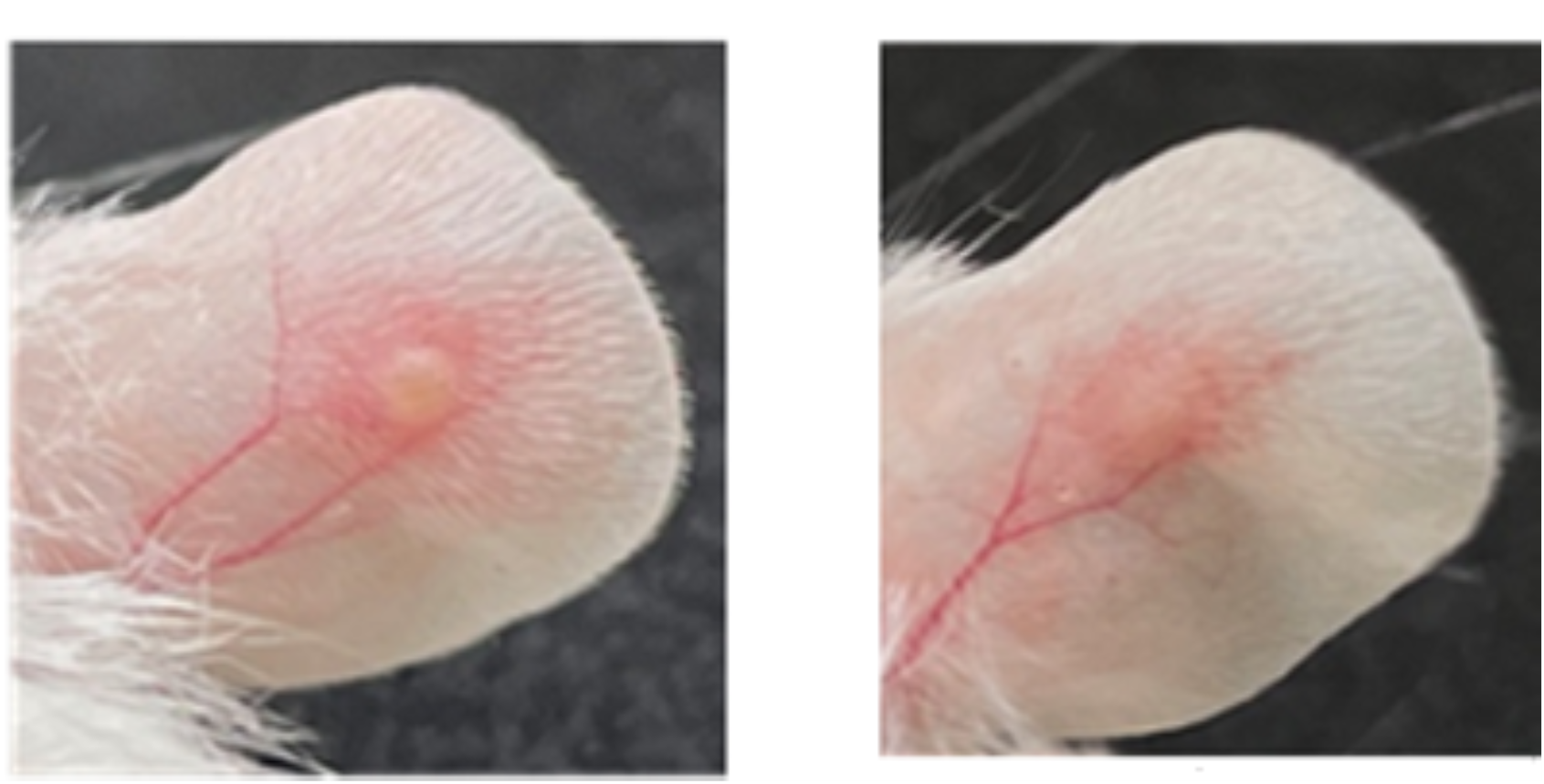
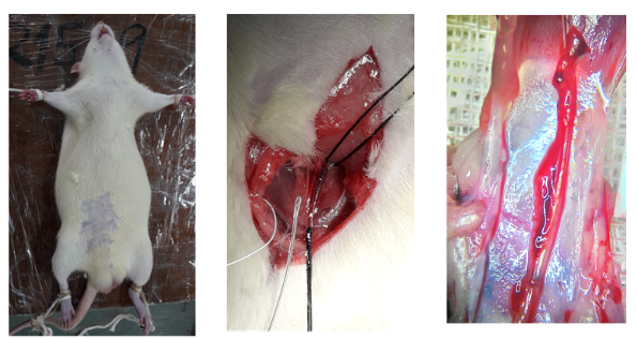
We collect clinical fibrin samples to investigate the role of bacteria-induced NETs in the development of catheter-related central venous stenosis and associated thrombosis.
We collect clinical samples to investigate the involvement of NETs in the pathogenesis of vaccine-induced adverse events.
We investigate the mechanisms underlying bacterial antibiotic resistance and explore alternative therapeutic agents targeting antibiotic-resistant pathogens, including vancomycin-resistant enterococci (VRE), multidrug-resistant Acinetobacter baumannii (MRAB), and multidrug-resistant Klebsiella pneumoniae (MRKP).
Our previous findings demonstrated that daptomycin treatment enhances MV production in VRE (Fig. 8), and that MVs, in turn, contribute to increased bacterial resistance against daptomycin (Fig. 9). We will continue to elucidate the molecular mechanisms underlying this phenomenon.
To develop alternative antimicrobial strategies against multidrug-resistant bacteria, we are screening antimicrobial peptides (AMPs). Several AMPs have been identified that exhibit potent activity against MRAB and MRKP in vitro and in vivo (Fig. 10). The underlying mechanisms of action and their potential for clinical application will be further investigated.
👥 Lab Members

- Dr. Chiau-Jing Jung, Associate Professor (Principal Investigator)
Research Assistants


Graduate Students






🤝 Collaborators

Dr. Yu-Min Kuo
Ph.D. in Clinical Medicine, College of Medicine, National Taiwan University; M.D., School of Medicine, National Taiwan University. Attending physician and clinical lecturer in the Department of Allergy, Immunology, and Rheumatology, Department of Internal Medicine, National Taiwan University Hospital. Specializes in autoimmune diseases and arthritis-related care.

Dr. Cheng-Wei Chen
Ph.D. in Clinical Medicine, College of Medicine, National Taiwan University; M.D., China Medical University. Attending surgeon and clinical assistant professor in the Department of Cardiovascular Surgery, Department of Surgery, National Taiwan University Hospital. Specializes in adult cardiac and peripheral vascular surgery.

Dr. Ming-Kai Chuang
M.D., School of Medicine, National Taiwan University. Attending physician in the Department of Laboratory Medicine, National Taiwan University Hospital. Specializes in acute myeloid leukemia and diagnostic hematology.

Dr. Yi-Hsien Shih
Ph.D. in Medical Sciences, Taipei Medical University; M.D., School of Medicine, National Taiwan University. Director of the Department of Dermatology, Shuang Ho Hospital. Specializes in atopic dermatitis, infectious skin diseases, and clinical dermatological treatment strategies.

Dr. Chih-Chieh Hsu
Ph.D. in Oral Biology, National Taiwan University; B.S. in Medical Laboratory Science and Biotechnology, Chang Gung University. Assistant Professor, Department of Microbiology, Soochow University. Research focuses on streptococcal pathogenesis and antibiotic resistance.
🏆 Research Projects and Journal Publications
Research Projects
Ongoing
- 2023/08 ~ 2026/07:Investigation of the Mechanisms and Regulation of Bacterial Extracellular DNA-Mediated Biofilm Formation in the Pathogenesis of Infective Endocarditis Caused by Streptococcus gordonii - National Science and Technology Council
- 2024/08 ~ 2025/07:Molecular Mechanisms of Neutrophil Extracellular Trap (NET) Formation Induced by Commensal Oral Streptococci and the Induction of Anti-dsDNA Antibodies via Molecular Mimicry (III) - National Science and Technology Council
Complete
- 2024/01 ~ 2024/12:Role and mechanism of the Type VII secretion system in immune evasion and biofilm formation by Streptococcus viridans in infective endocarditis - 校內計畫
- 2023/08 ~ 2024/07:Molecular mechanisms of commensal oral streptococci-induced NETosis and cross-reactivity leading to anti-dsDNA antibody production (2/3) - National Science and Technology Council
- 2023/07 ~ 2024/02:Investigating the role of pigment production in the virulence and pathogenesis of Streptococcus mutans - National Science and Technology Council
- 2022/08 ~ 2023/07:Regulatory roles of two-component systems in platelet-dependent biofilm formation and antibiotic resistance in Streptococcus gordonii-induced endocarditis - National Science and Technology Council
- 2022/07 ~ 2023/02:Investigating the role of bacterial pigment in platelet aggregation and biofilm formation by Streptococcus mutans - National Science and Technology Council
- 2022/07 ~ 2023/02:Functional analysis of VicR regulator in the interaction between Streptococcus gordonii and platelets - National Science and Technology Council
- 2021/08 ~ 2022/07:Roles and mechanisms of BSP-like repeat family proteins in immune evasion and biofilm formation by Streptococcus viridans in infective endocarditis (3/3) - National Science and Technology Council
- 2021/07 ~ 2022/02:Role of PrsA protein in infective endocarditis caused by Streptococcus viridans - National Science and Technology Council
- 2020/08 ~ 2021/07:Roles and mechanisms of BSP-like repeat family proteins in immune evasion and biofilm formation by Streptococcus viridans in infective endocarditis (2/3) - National Science and Technology Council
- 2020/07 ~ 2021/02:Role of ARP-42 protein in phagocytosis resistance and biofilm formation in Streptococcus mutans - National Science and Technology Council
- 2019/08 ~ 2020/07:Roles and mechanisms of BSP-like repeat family proteins in immune evasion and biofilm formation by Streptococcus viridans in infective endocarditis (1/3) - National Science and Technology Council
- 2018/08 ~ 2019/07:Role of autolysin regulatory machinery in Streptococcus mutans survival and biofilm formation in infective endocarditis (2/2) - National Science and Technology Council
- 2017/08 ~ 2018/07:Role of autolysin regulatory machinery in Streptococcus mutans survival and biofilm formation in infective endocarditis (1/2) - National Science and Technology Council
- 2017/04 ~ 2018/03:New Faculty Research Grant - Startup Funding Program
Journal Publications
📍 Contact
Address: No. 250, Wuxing Street, Xinyi District, Taipei City, Taiwan
Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University
TEL: +886-2-2736-1661 # 27101
Email: cjjung@tmu.edu.tw



